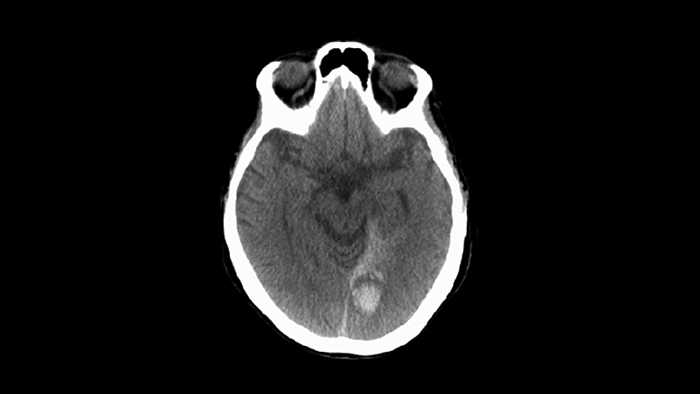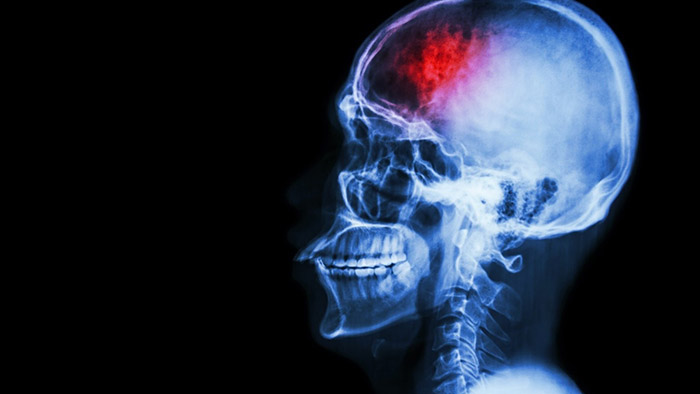
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced a major clinical trial to assess the impact of a ‘Direct to Angio Suite’ workflow on stroke patient outcomes. The study will assess whether with Philips’ advanced image-guided therapy platform it is possible to diagnose, plan and treat stroke patients in the interventional suite without requiring an initial CT or MRI exam. The multicenter, prospective, randomized, controlled, open-label, blinded-endpoint trial will be run across eight sites and over 460 patients globally. It will begin in the first half of next year and is expected to be completed in 2022. Outcomes for stroke patients are closely tied to how quickly they receive treatment: every 30 minutes’ delay before treatment reduces the chance of a good outcome by 14% [1], and every hour of delay ages the brain by 3.6 years compared to a normally aging brain [2]. Currently, when a possible stroke patient arrives at the Emergency Department, they typically first undergo a CT or MRI exam and, in the case of an ischemic stroke, are then treated in an interventional suite. Several studies have indicated that a Direct to Angio Suite workflow can reduce the time to treatment and improve patient outcomes. Philips is developing new technology to further improve the CT-like images of the brain created with the X-ray system in the suite. The WE-TRUST (Workflow optimization to rEduce Time to endovascular ReperfUsion in Stroke Treatment) trial will provide the most comprehensive assessment to-date of the impact of this technology and workflow innovation on time to treatment and patients’ neurological outcomes. “Significant advances in technology mean that we are now able to identify, plan and treat ischemic stroke patients in the angiography suite, without the need for a separate CT or MR scan,” said Raul G. Nogueira, MD, Director, Neuroendovascular Service Marcus Stroke & Neuroscience Center at Grady Memorial Hospital in Atlanta, U.S. and principal investigator for the WE-TRUST trial. “Now is the right moment to perform a randomized controlled trial to objectively assess the benefits of a streamlined Direct to Angio Suite workflow on patient outcomes.”
For stroke patients, ‘time is brain’. The WE-TRUST trial will assess the impact of a streamlined Direct to Angio Suite workflow on patient outcomes and has the potential to make a significant impact in this rapidly advancing field.
Ronald Tabaksblat
General Manager Image Guided Therapy Systems at Philips
“With extensive clinical research demonstrating the benefit of a treatment approach that combines thrombectomy and clot-busting drugs, stroke patient triage and treatment has changed dramatically in recent years,” said Ronald Tabaksblat, General Manager Image Guided Therapy Systems at Philips. “For stroke patients, ‘time is brain’. The WE-TRUST trial will assess the impact of a streamlined Direct to Angio Suite workflow on patient outcomes and has the potential to make a significant impact in this rapidly advancing field.” Philips provides a unique portfolio of systems, smart devices, software and services in image-guided therapy, which combine to provide healthcare providers with sophisticated, procedure-oriented solutions.
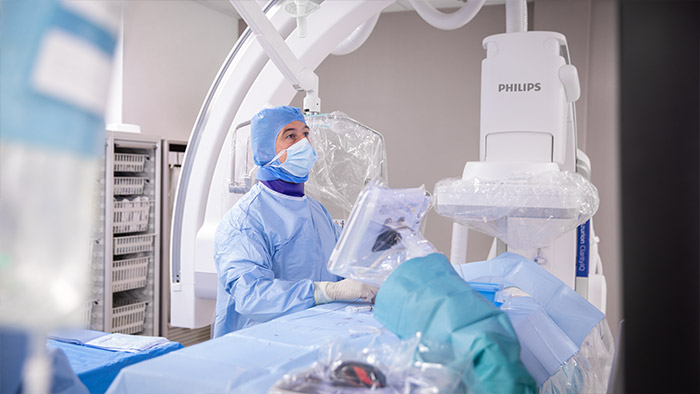
The trial will primarily be carried out on Philips Azurion, the company’s leading platform for interventional procedures, at eight leading stroke sites in Europe and the US. The primary endpoint of the WE-TRUST trial is patients’ cognitive function at three months after the procedure. More information about the Direct to Angio Suite workflow can be found at philips.com/dtas.